
Appointment Prep
Communicating openly and sharing with your doctor how your condition is affecting you from day to day is an important part of helping to assess your current treatment plan. Your appointment is an opportunity to share symptoms, ask questions, and discuss your goals. The tools and resources below may help you explore the impact of your condition, and share tips designed to encourage communication with your healthcare provider.
For adults with moderate to severe rheumatoid arthritis when TNF blockers did not work well or could not be tolerated.
ra_token: AEM-xeljanz_uat-DDG-RA-pdf-config-download
ra_tokenMail: AEM-xeljanz_uat-DDG-RA-pdf-config
ra_tokenImage: xeljanz_uat-DDG-RA-Image-config-download
ra_tokenMailImage: xeljanz_uat-DDG-Image-RA-pdf-config
psa_token: AEM-xeljanz_uat-DDG-PSA-pdf-config-download
psa_tokenMail: AEM-xeljanz_uat-DDG-PSA-pdf-config
ra_token: form_builder__production__578__wd
ra_tokenMail: form_builder__production__590__wd
ra_tokenImage: form_builder__production__1133__wd
ra_tokenMailImage: form_builder__production__1263__wd
psa_token: form_builder__production__600__wd
psa_tokenMail: form_builder__production__593__wd
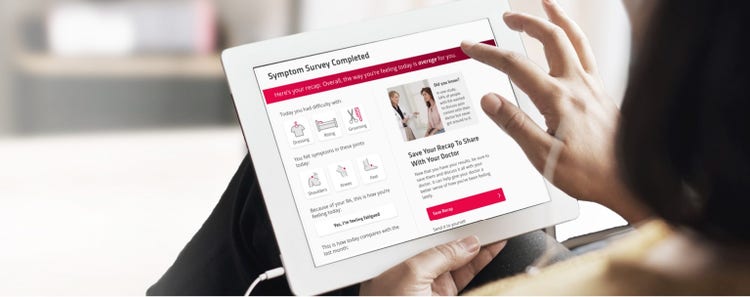
This survey is provided for educational purposes only and is not intended to replace discussions with a healthcare provider. This is not intended to be used as a diagnostic tool. Decisions regarding patient care must be made with a healthcare provider, considering the unique characteristics of the patient.

Did you know?
In one study, 54% of people with RA wanted to discuss pain control with their doctor but never got around to it.
For adults with active psoriatic arthritis when TNF blockers did not work well or could not be tolerated.

ra_token: AEM-xeljanz_uat-DDG-RA-pdf-config-download
ra_tokenMail: AEM-xeljanz_uat-DDG-RA-pdf-config
psa_token: AEM-xeljanz_uat-DDG-PSA-pdf-config-download
psa_tokenMail: AEM-xeljanz_uat-DDG-PSA-pdf-config
psa_tokenImage: xeljanz_uat-DDG-PSA-Image-config-download
psa_tokenMailImage: xeljanz_uat-DDG-PSA-Image-config
ra_token: form_builder__production__578__wd
ra_tokenMail: form_builder__production__590__wd
psa_token: form_builder__production__600__wd
psa_tokenMail: form_builder__production__593__wd
psa_tokenImage: form_builder__production__1264__wd
psa_tokenMailImage: form_builder__production__1266__wd
This survey is provided for educational purposes only and is not intended to replace discussions with a healthcare provider. This is not intended to be used as a diagnostic tool. Decisions regarding patient care must be made with a healthcare provider, considering the unique characteristics of the patient.

Did you know?
In one study, 41% of people with PsA have not met their therapy goals with their current treatment.
For adults with moderate to severe ulcerative colitis when TNF blockers did not work well or could not be tolerated.
Is Your Approach To Life With UC All That It Can Be?
Living with UC isn’t easy, but don’t be discouraged. There are things you can do to help manage your condition. Answer a few questions about how UC is impacting your life and then discuss your disease and treatment plan with your gastroenterologist (GI).

token: AEM-xeljanz_uat-DDG-UC-pdf-config-download
tokenMail: AEM-xeljanz_uat-DDG-UC-pdf-config
token: form_builder__production__609__wd
tokenMail: form_builder__production__610__wd
All content on this website is for informational purposes only. The content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of a physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay seeking it because of something you have read on this website.
Doctor Discussion Videos
Watch as experts share their thoughts on how to improve communication between you and your doctor.
Preparing for a Doctor Appointment
Dr. Dana Lukin, MD
In the next video, learn about being your own best advocate when talking to your doctor

Being Your Own Best Advocate
Dr. Grace Wright, MD, PhD, FACR
In the next video, learn about the importance of communication with your doctor

Supporting Good Communication
Dr. Dana Lukin, MD
In the next video, learn why honesty is always the best policy with your doctor

Tips For Your Appointment
Doctors may offer patients the option to schedule in-office or virtual visits. These tips may be helpful on your next appointment—whether you’ll be at the doctor’s office or on your device.
If you aren’t sure which telemedicine providers or services your insurance plan covers, call your insurance provider before scheduling your appointment.
In-Office
Plan ahead
It’s important to be seen by your doctor regularly. Schedule in-office visits as far in advance as possible for the best choice of options that match your schedule.
Practice your conversation
Go over the topics you want to discuss with your doctor in advance. Practicing with a family member or friend may help you build confidence.
Bring a notebook
In addition to bringing the questions you want to ask your doctor, you may consider taking notes during your visit to help you remember the conversation after you leave.
Speak up
Don’t hesitate to advocate for yourself, especially if you're not feeling enough relief with your current treatment.
Virtual
Test your tech
Set up and double-check the devices you plan to use for your appointment to make sure everything works. Consider asking a tech-savvy family member or caregiver for help.
Protect your privacy
Find a space where you can speak openly and consider using a headset or earbuds for extra privacy.
Get comfortable
Select an area where you feel at ease and can focus without too many distractions.
Prepare in advance
Think about what you’d like to share with your doctor. Have questions ready and take notes you can reference later.
